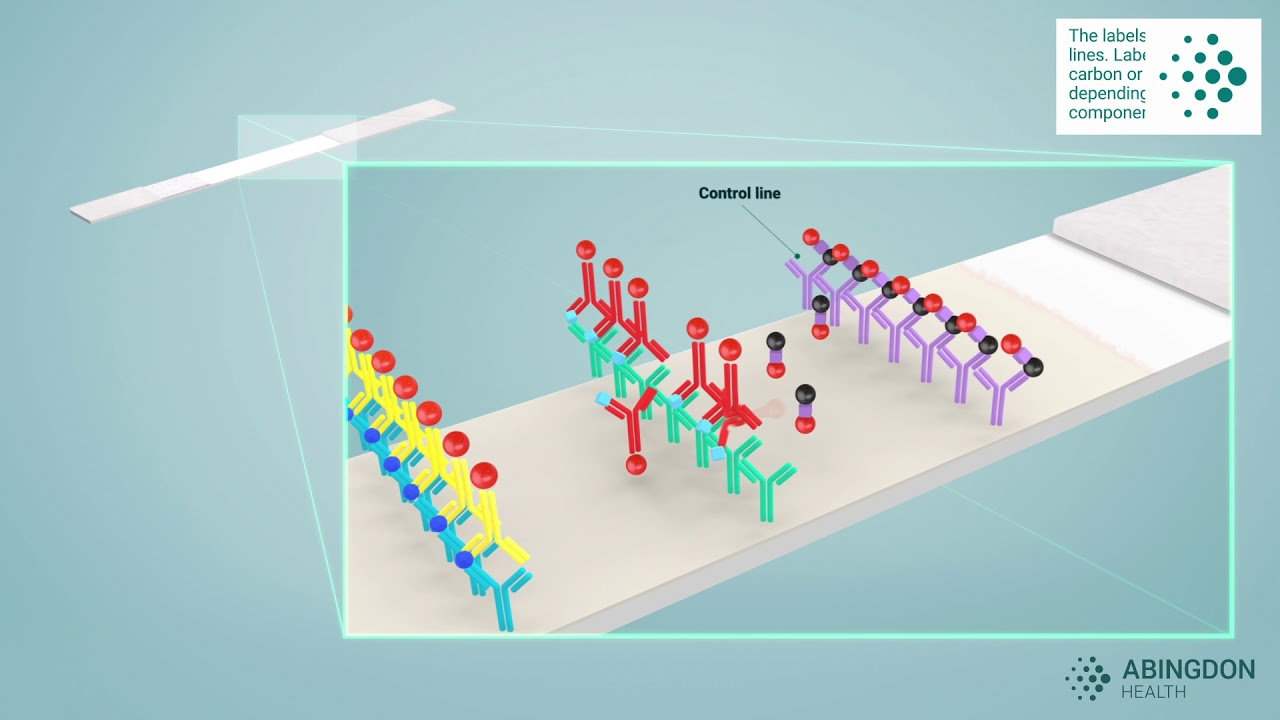
lateral flow assay development
A prototype of a rapid lateral flow assay for antibody to A. Choosing the right sample pad conjugate release pad and nitrocellulose membrane is critical to developing sensitive and reliable lateral flow assays.

Enhancing Sensitivity Of Lateral Flow Assay With Application To Sars Cov 2 Applied Physics Letters Vol 117 No 12
Published Nov 2 2022.

. The same structure is used whether the. Soybean mosaic virus SMV is the most common virus in soybean and poses a serious threat to crop production and germplasm recession in many countries worldwide. Lateral Flow Assay Services.
Through tried and tested. Lateral flow rapid test development at Abingdon Health typically follows a number of stages with formal reviews at critical points. The Core Assay Development Starter Kit comes with all that you need to begin optimizing your assay development.
Start with sticking the. In this study a highly. Core Assay Development Starter Kit includes.
Lumos manages the full development program for point-of-care POC assays from sourcing andor generation of reagents right through to verification and. Standardizing membrane characteristics and optimizing molecular level immunoassay reaction. The Lateral Flow Assay Components market report is highly structured into a region-wise study.
Many years of developing lateral flow assays for clients and having launched our own products means we recognise the pressures of developing new rapid tests. Chapter 9 Development Trend of Lateral Flow Assay 2022-2030 91 Global Lateral Flow Assay Market Size Sales and Revenue Forecast 2022-2030. 50 Nitrocellulose An.
Lateral flow assays LFAs are rapid and inexpensive diagnostic devices that can be used to test for a target substance analyte in a sample. Bio-Techne is a leading provider of bio-reagents and analytical instrumentation and now provides lateral flow assay. The first infectious disease lateral flow assays were commercialized in the late 1980s identifying the presence of Group A Streptococcus pyogenes collected with.
Lateral Flow Assay Development Guide Lateral flow assays LFAs are rapid and inexpensive diagnostic devices that can be used to test for a target substance analyte in a sample. Taking a lateral flow test strip from the design stage through product development to final manufacturing is a process that employs principles from biology. Lateral flow assay development is the successful production of a simple to use diagnostic test for validating the presence or absence of a wide range of pathogens biomarkers and.
Now you need to stick all the pads on the lateral flow backing card. Bio-Techne is a leading provider of bio-reagents and analytical instrumentation and now provides lateral flow assay development services including. If you are developing a new point-of-care assay or.
We have a wealth of expertise in the development of highly responsive point-of-care lateral flow assays with over 250 successful projects. We have developed a large range of lateral flow assay from fully quantitative reader based assays to complex multiplex qualitative assays as well as the simplest two line qualitative assay. It will ensure the right performance of lateral flow assay development.
The regional analysis comprehensively done by the researchers highlights. Lateral flow immuno-chromatographic assays commonly known as lateral flow assays are devices used in laboratories to detect the. The development of Lateral Flow Immunochromatography Assay can be divided into two levels.
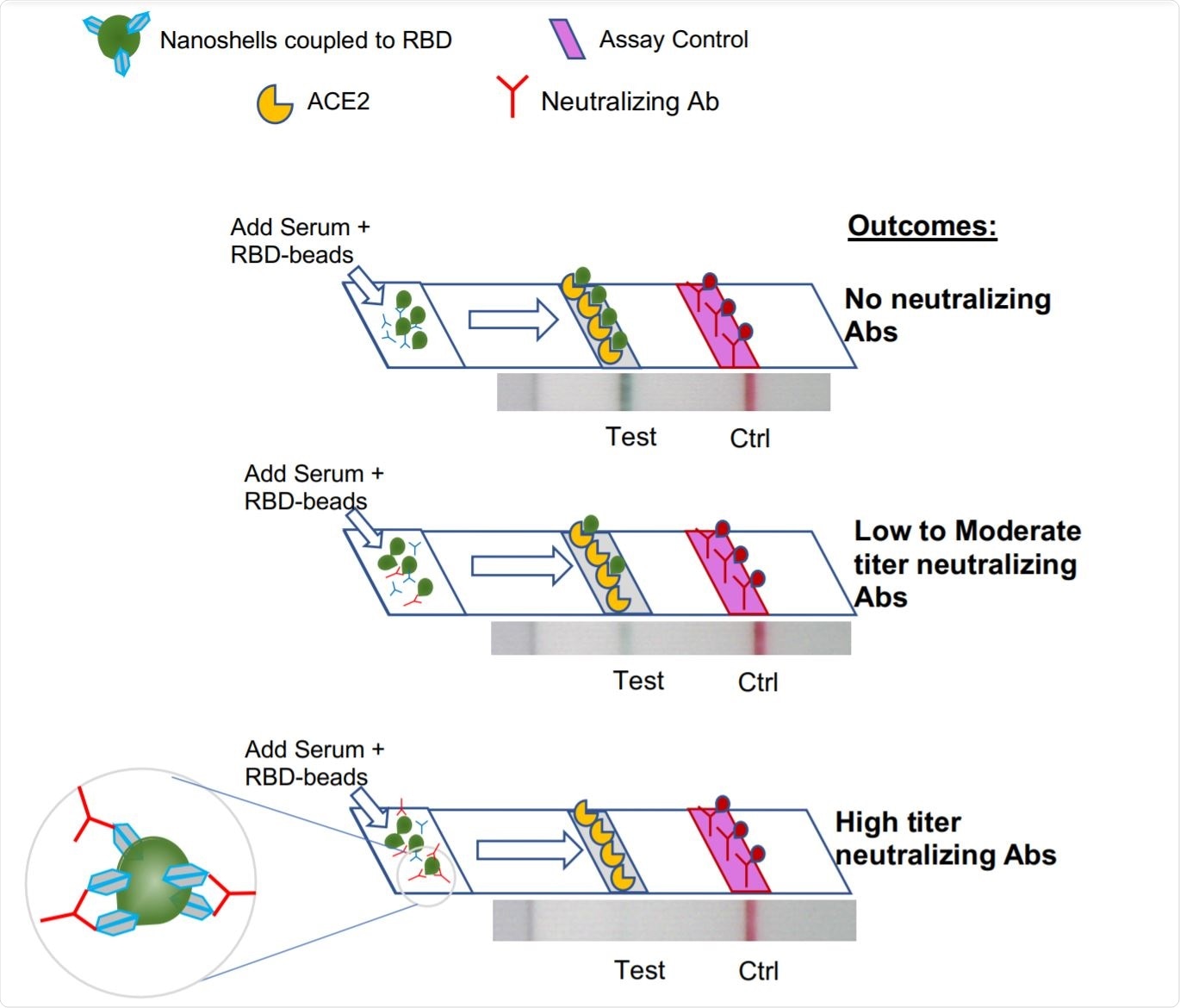
Rapid Point Of Care Lateral Flow Assay To Assess The Neutralizing Activity Of Plasma Against Sars Cov 2

Pdf Development Of Chemiluminescent Lateral Flow Assay For The Detection Of Nucleic Acids Semantic Scholar
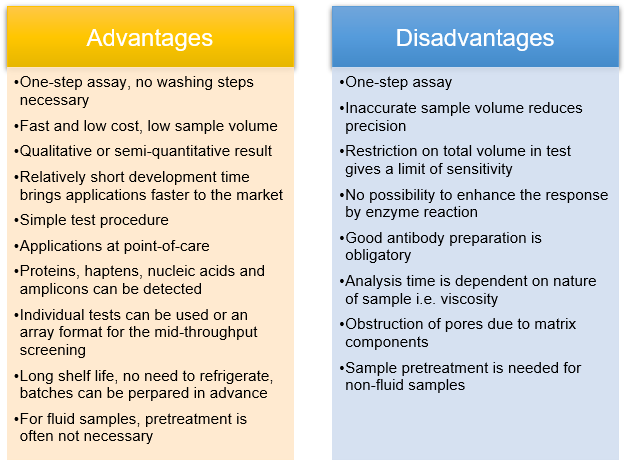
Lateral Flow Assay Cd Bioparticles

Universal Lateral Flow Assay Kit Ab270537 Abcam
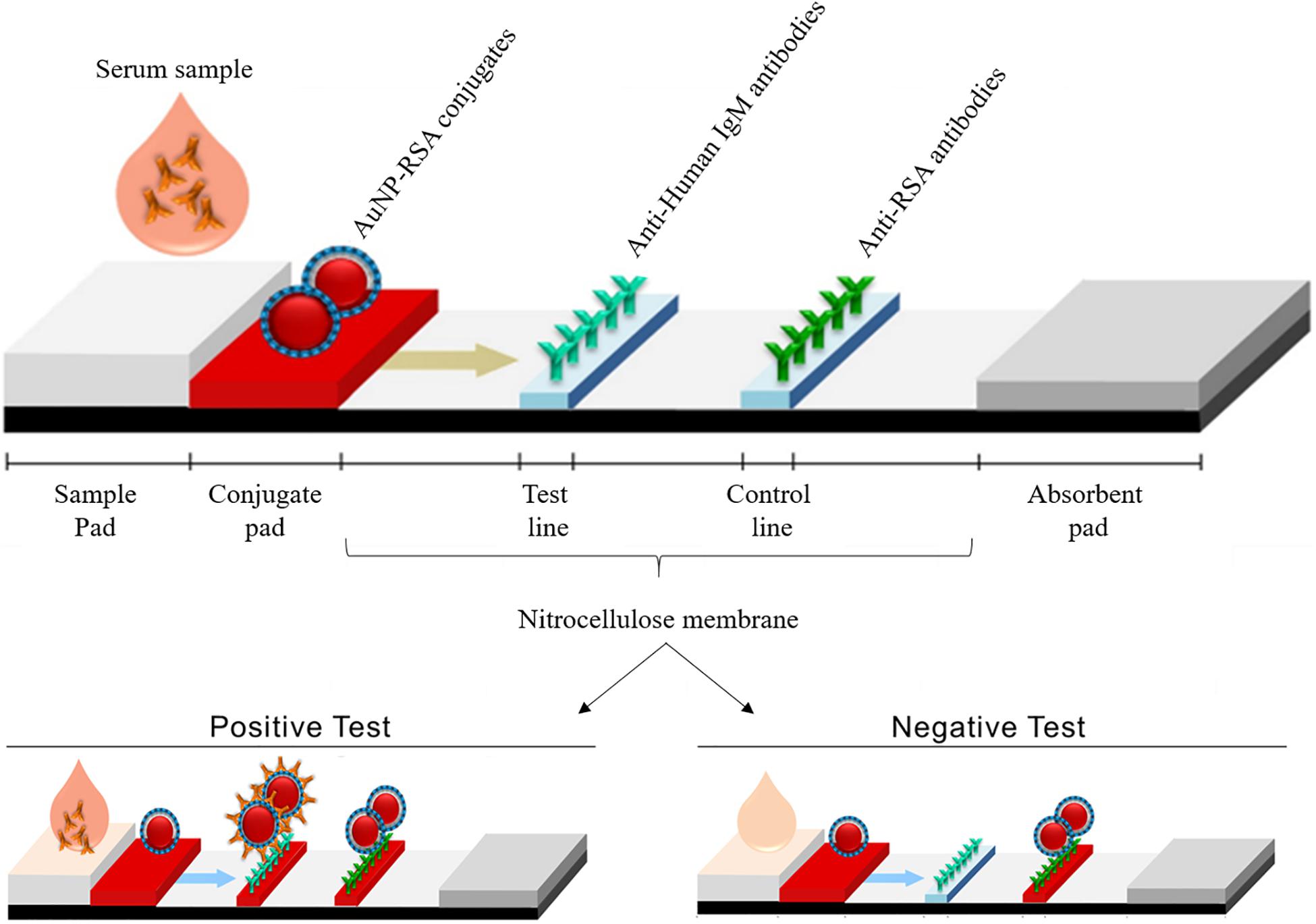
Frontiers Development Of A Gold Nanoparticle Based Lateral Flow Immunoassay For Pneumocystis Pneumonia Serological Diagnosis At Point Of Care
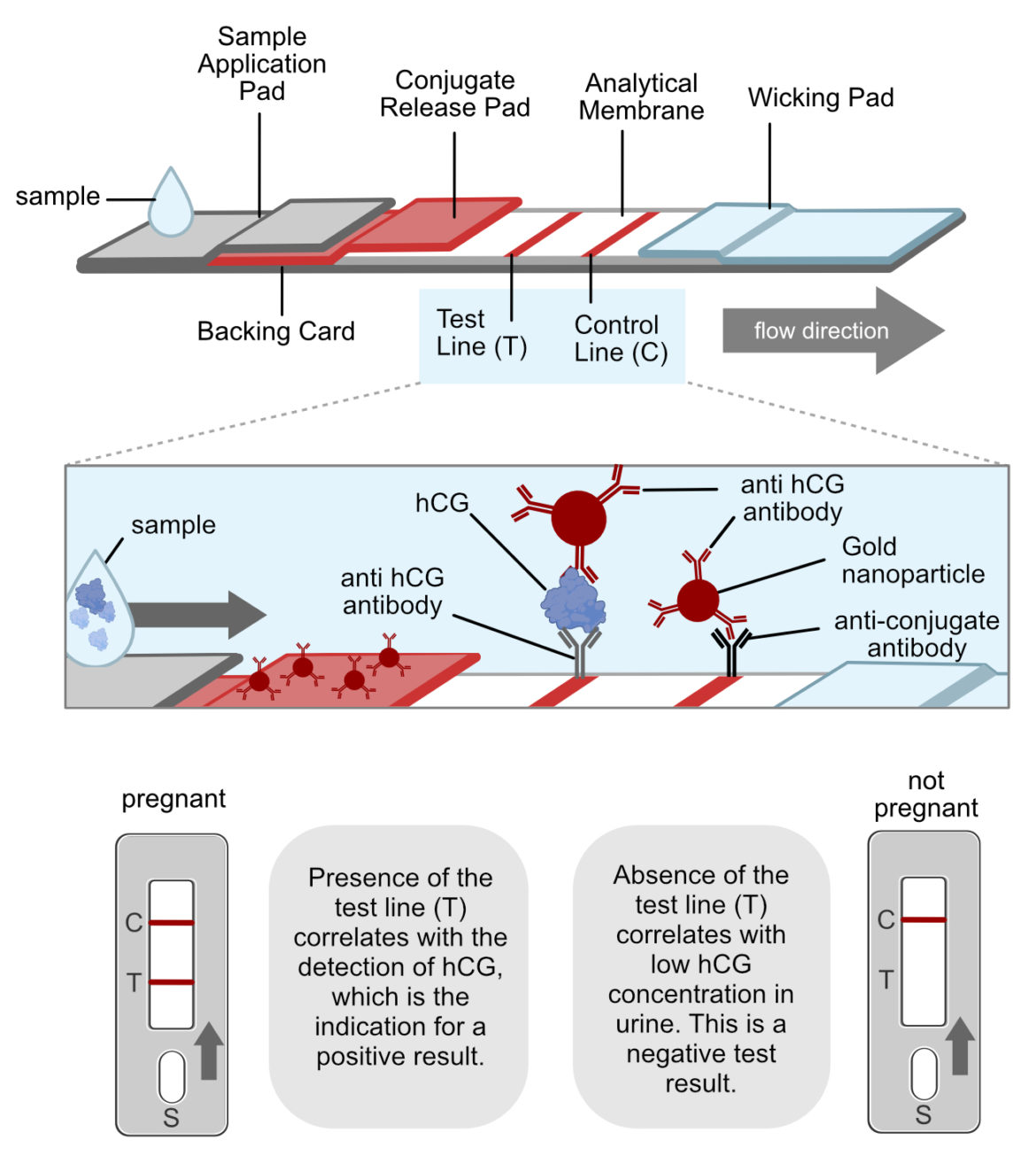
Milenia Hybridetect Lateral Flow Development Platform
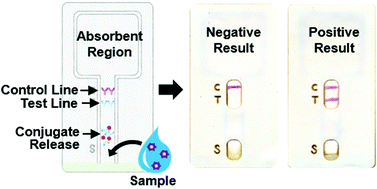
Lateral Flow Immunochromatographic Assay On A Single Piece Of Paper Analyst Rsc Publishing
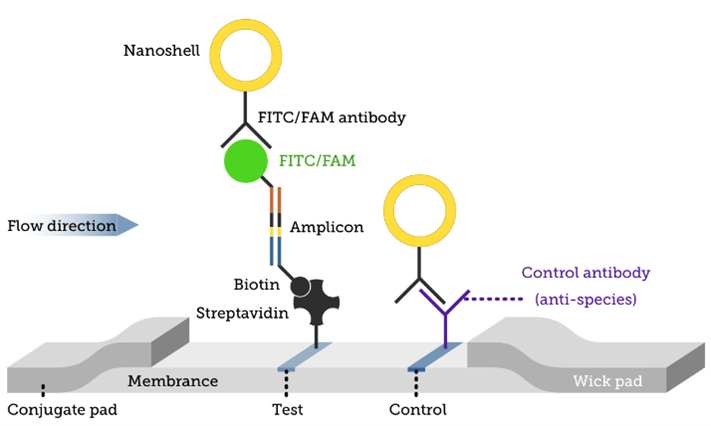
Lateral Flow Assay Products Services Fortis Life Sciences
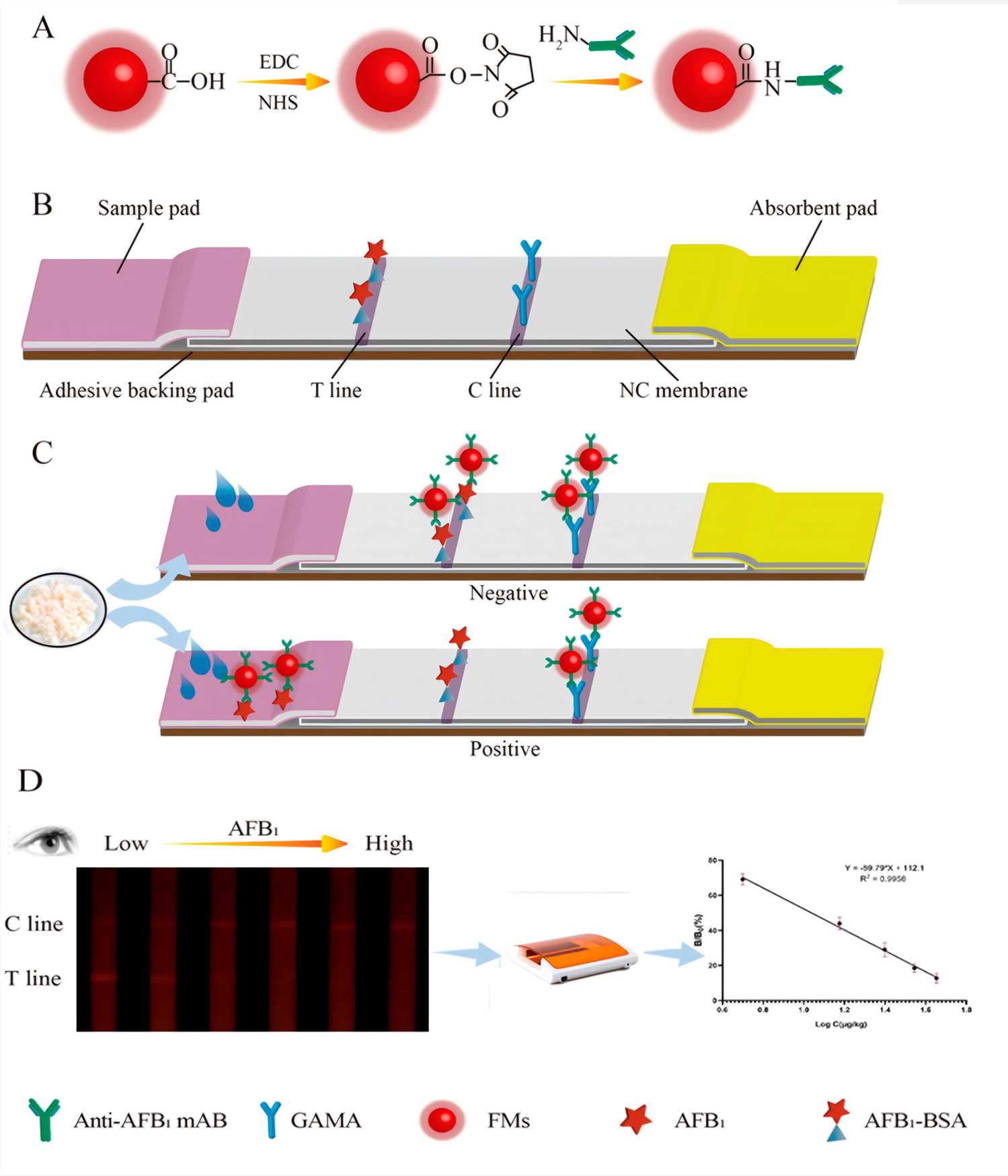
Lateral Flow Immunochromatographic Assay Lfia Based Kits Development Creative Biolabs
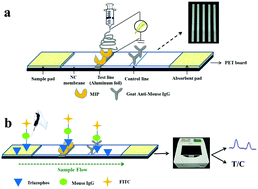
Development Of Fluorescent Lateral Flow Test Strips Based On An Electrospun Molecularly Imprinted Membrane For Detection Of Triazophos Residues In Tap Water New Journal Of Chemistry Rsc Publishing
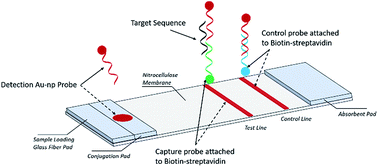
Simple Lateral Flow Assays For Microbial Detection In Stool Analytical Methods X Mol

Lateral Flow Assays Principles Designs And Labels Sciencedirect

Dcn Diagnostics Lateral Flow Assay Development Youtube
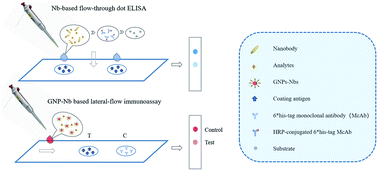
Development Of Nanobody Based Flow Through Dot Elisa And Lateral Flow Immunoassay For Rapid Detection Of 3 Phenoxybenzoic Acid Analytical Methods Rsc Publishing

Pdf Development Of Multiplexed Infectious Disease Lateral Flow Assays Challenges And Opportunities Semantic Scholar

Lateral Flow Assay Development Kit For Lateral Flow Development

Lateral Flow Assays And Applications Joysbio Biotechnology
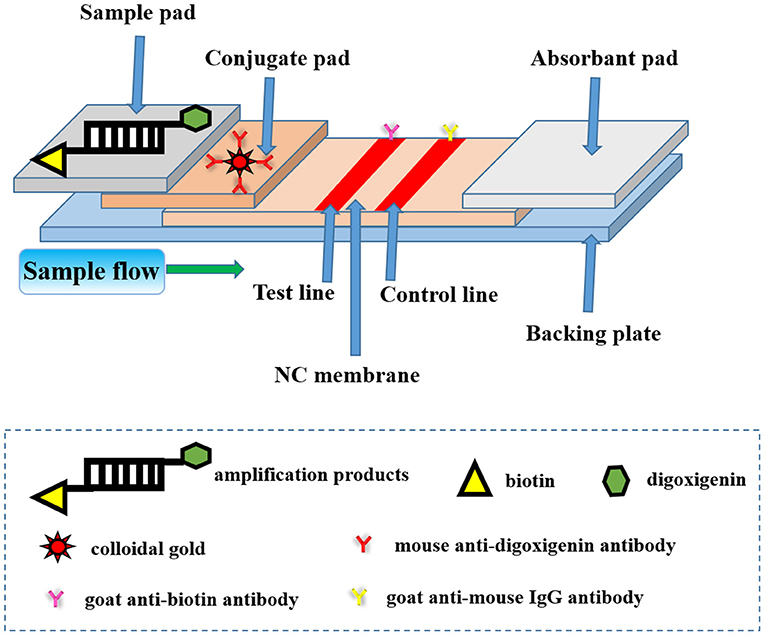
Frontiers A Novel Lateral Flow Assay For Rapid And Sensitive Nucleic Acid Detection Of Avibacterium Paragallinarum